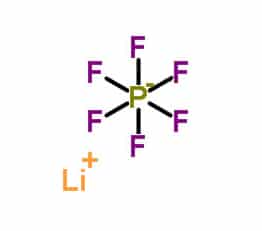
LiPF6, or Lithium hexafluorophosphate, is an inorganic compound with the chemical formula LiPF6. It is one of the most commonly used electrolyte salts in lithium-ion batteries, primarily due to its good solubility in organic solvents and high ionic conductivity.
Key Properties:
- High Ionic Conductivity: LiPF6 effectively dissociates in organic solvents (such as ethylene carbonate, dimethyl carbonate, etc.), providing high ionic conductivity, which is crucial for the efficient operation of lithium-ion batteries.
- Electrochemical Stability: LiPF6 exhibits good electrochemical stability over a wide voltage range, making it suitable for high-voltage lithium-ion batteries.
- Thermal Stability: Although LiPF6 decomposes at high temperatures, it remains relatively stable at room temperature, making it suitable for most lithium-ion battery operating environments.
Applications:
- Lithium-Ion Batteries: LiPF6 is a key component of the electrolyte in lithium-ion batteries, widely used in consumer electronics, electric vehicles, and energy storage systems.
- Other Electrochemical Devices: LiPF6 can also be used in other applications requiring high ionic conductivity and electrochemical stability.
| Chemical Name | Lithium Hexafluorophosphate |
| CAS# | 21324-40-3 |
| Molecular Appearanceula | LiPF6 |
| Assay | >99.9% |
| Moisture | <50 ppm |
| Acid (HF) | <100 ppm |
| Molecular weight | 151.91 |
| Melting point | ℃ |
| Density | g/mL |
| Appearance | White powder |