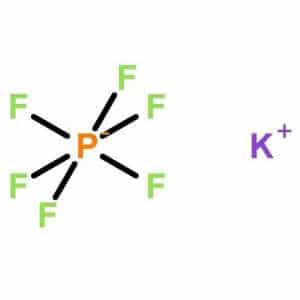
KPF6, or Potassium hexafluorophosphate, is an inorganic compound with the chemical formula KPF6. It is a salt composed of potassium cations (K⁺) and hexafluorophosphate anions (PF6⁻). KPF6 is known for its stability and low reactivity, making it useful in various chemical and electrochemical applications.
Key Properties:
- Solubility: KPF6 is soluble in water and some organic solvents, though its solubility is generally lower compared to LiPF6.
- Stability: The hexafluorophosphate anion (PF6⁻) is chemically and thermally stable, making KPF6 a robust compound.
- Non-coordinating Anion: PF6⁻ is a weakly coordinating anion, which means it does not strongly interact with other ions or molecules, making it useful in catalysis and electrochemical studies.
Applications:
- Electrochemistry: KPF6 is used as an electrolyte salt in some electrochemical systems, particularly in non-aqueous electrolytes.
- Chemical Synthesis: It is used as a source of the PF6⁻ anion in organic and inorganic synthesis, often as a counterion to stabilize cationic complexes.
- Ionic Liquids: KPF6 can be used in the preparation of ionic liquids, which are useful in various industrial and research applications.
- Catalysis: Due to the weakly coordinating nature of PF6⁻, KPF6 is sometimes used in catalytic systems where minimal interference from the anion is desired.
Comparison with LiPF6:
- While LiPF6 is widely used in lithium-ion batteries, KPF6 is less common in battery applications due to the larger size and lower mobility of the potassium ion (K⁺) compared to lithium (Li⁺).
- KPF6 is more commonly used in chemical synthesis and research rather than in energy storage systems.
| Chemical Name | Lithium Hexafluorophosphate |
| CAS# | 17084-13-8 |
| Molecular Appearanceula | KPF6 |
| Assay | >99.9% |
| Moisture | <500 ppm |
| Acid (HF) | <100 ppm |
| Molecular weight | 184.06 |
| Melting point | ℃ |
| Density | g/mL |
| Appearance | White powder |