A conventional electrolytic cell is a type of electrochemical cell that is used to carry out electrolysis. Electrolysis is when an electric current is passed through a solution containing ions, causing the ions to migrate toward opposite electrodes and undergo chemical reactions at those electrodes.
A conventional electrolytic cell consists of two electrodes, an anode and a cathode, that are placed in an electrolyte solution. The anode is the electrode where oxidation (loss of electrons) takes place, while the cathode is the electrode where reduction (gain of electrons) takes place. The two electrodes are separated by a diaphragm or a membrane to prevent the mixing of the products of the two reactions.
When a voltage is applied to the electrodes, an electric current flows through the solution and causes the positively charged ions to move toward the cathode and the negatively charged ions to move toward the anode. At the cathode, the positively charged ions accept electrons and are reduced to form neutral atoms or molecules, while at the anode, the negatively charged ions lose electrons and are oxidized to form neutral atoms or molecules.
Conventional electrolytic cells are used in various industrial processes, such as the production of aluminum, chlorine, and hydrogen gas. They are also used in electroplating, which is depositing a thin layer of one metal onto another metal surface.
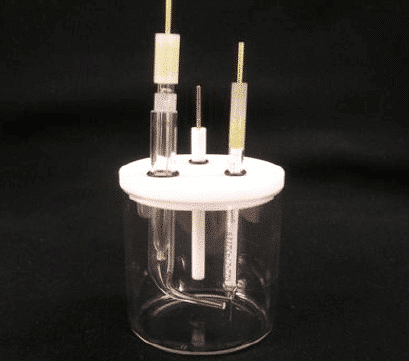
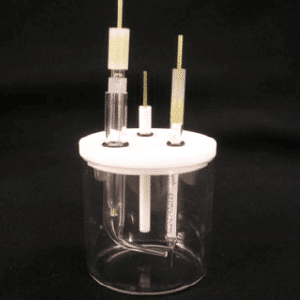
Glass pool, PTFE cover, not sealed, 100mL/250mL/500mL



Reviews
There are no reviews yet.